While I worked in several different countries in South and Central America as a graduate student and postdoc, I never worked in Peru. This was unfortunate, because Peru is one of the most biodiverse countries on earth, with an unrivaled diversity of biogeographic regions and habitats. The diversity of life in the forests that climb into the foothills of the Andes from the Amazon basin is simply astounding. This is especially true for poison frogs, with a plethora of species in the northern parts of the Amazon-Andes transition zone of Peru.

I first ventured to Peru in 1999 on the invitation of Rainer Schulte, a German who went to Peru to pursue graduate research in herpetology, but ended up settling in Peru, where he remains to this day, in the city of Tarapoto. My first trip to Peru was a revelation, as Rainer and I traveled all over north central Peru, seeing some of the astonishing diversity of the poison frogs of this region. The scenery was also pretty spectacular, with dense rainforest covering the huge, almost vertical slopes of the mountains.
Rainer had brought a world war II vintage vehicle, called a Unimog, from Germany. This huge truck had gigantic wheels and could go almost anywhere. Here (below) is the unimog driving through the valley between Tarapoto and the Amazon basin.
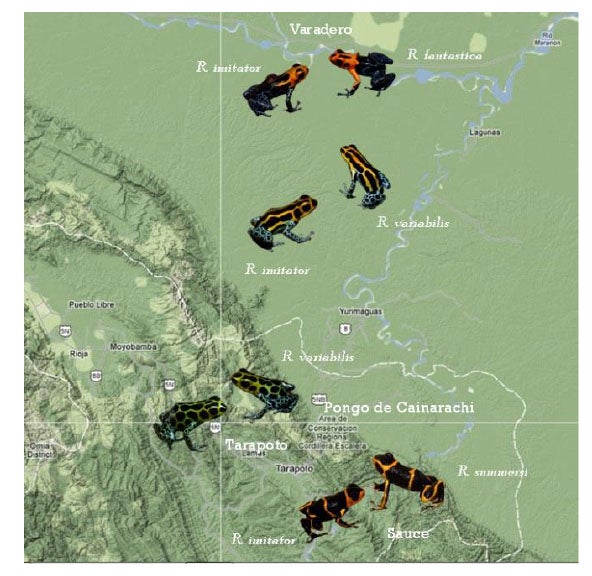
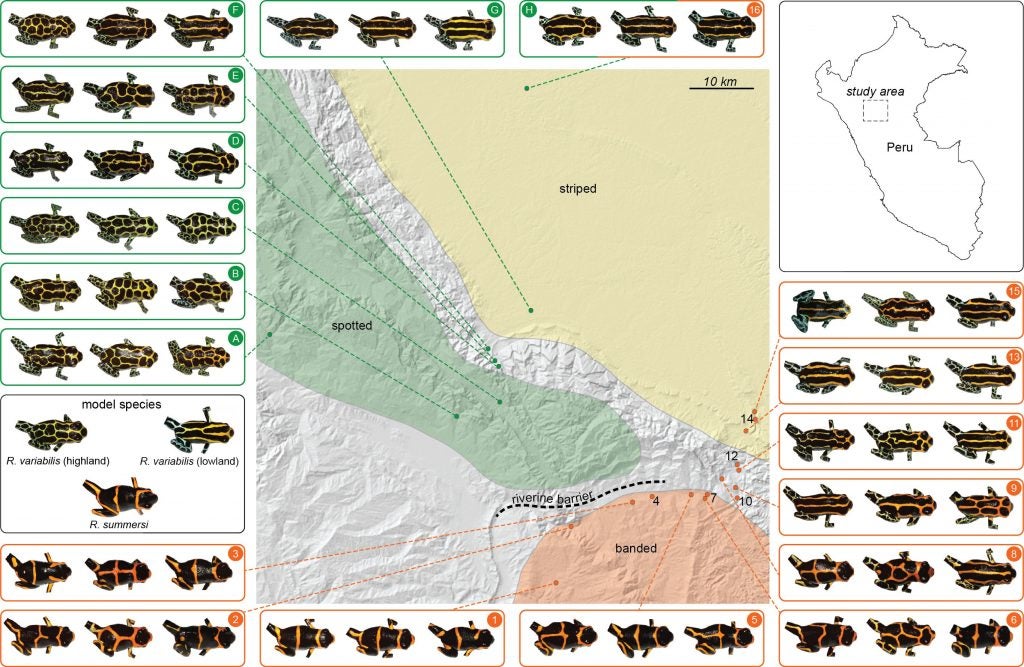
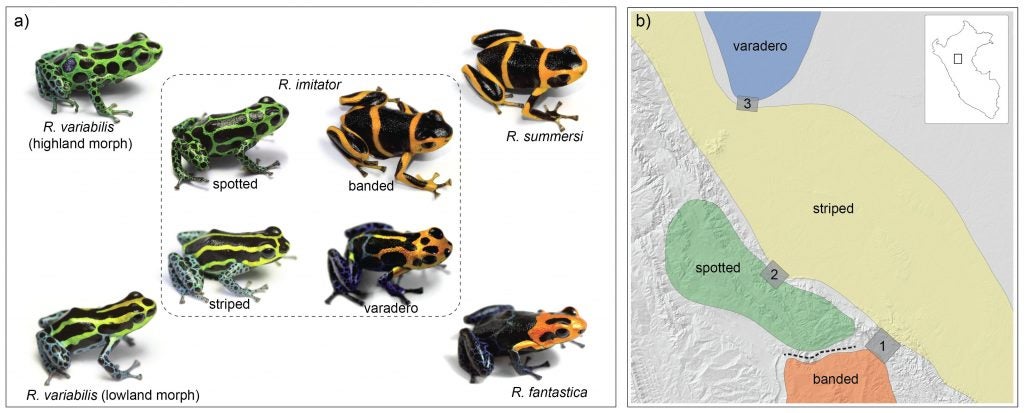
This region is home to a variety of beautiful poison frogs. One of the most intriguing is Ranitomeya imitator, the mimic poison frog, as it resembles different species of poison frog in different places. For example, this spotted color pattern morph from the mountains near Tarapoto and San Jose closely resembles this spotted form of Ranitomeya variabilis, a related species that lives in the same mountains.
There are many other species as well, such as the following. Ranitomeya fantastica is sympatric with R. imitator and R. variabilis, but seems to be rarer, or harder to find (they may be more arboreal, which could be related to this).
Ameerega cainarachi is often found near streams
Amereega bassleri comes in a wide variety of color patterns, some of which are shown below.
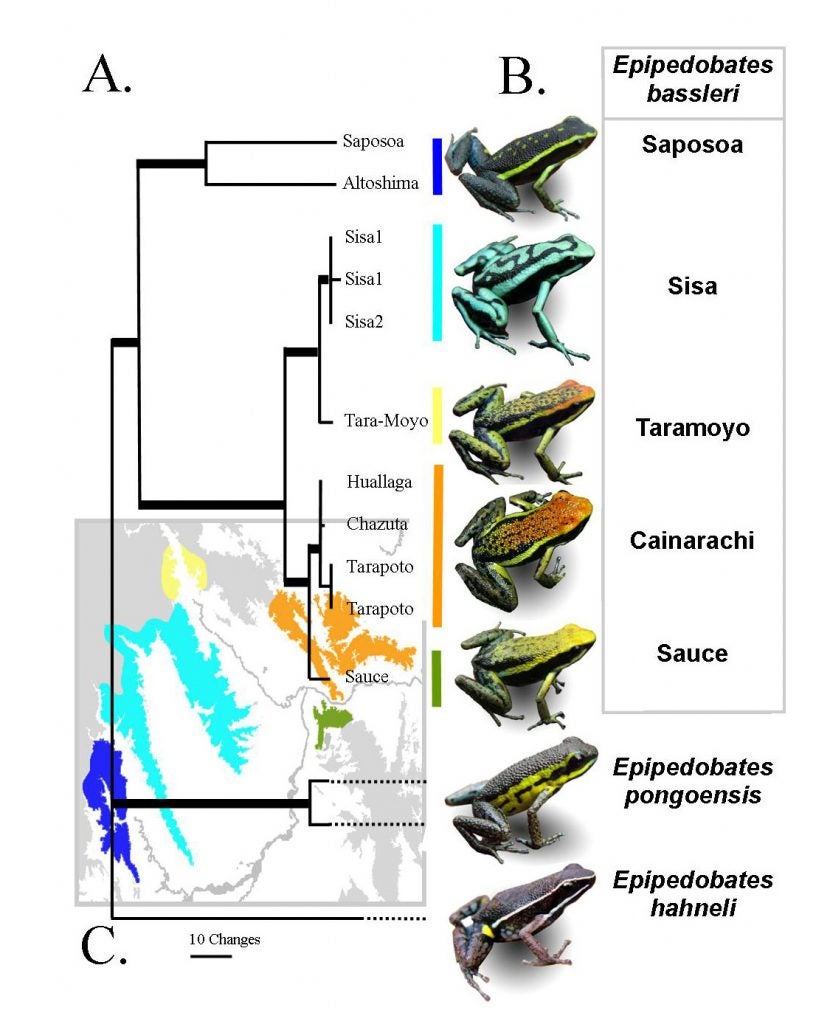
My former graduate student Jenny Roberts and I spent time in Peru collecting various species of Ameerega and various morphs of A. bassleri for phylogenetic analyses (Roberts et al. 2006). The different color morphs of A. bassleri live on different montane ridges (“sky islands”) in San Martin, Peru (Roberts et al. 2007). Note that the genus name has changed.
Later, Jason Brown and Evan Twomey followed up with more complete sampling of frogs of the genus Ameerega, discovering a number of new species in the process (Brown & Twomey 2009 Zootaxa).
There are also some distantly related mimics of these poison frogs, such as this Lithodytes lineatus, which may be a Batesian mimic, or possibly a Mullerian mimic, of striped species like E. hahneli.
If one journeys directly east of Tarapoto, one comes to the lowlands around Pongo de Cainarache. This small town is near several forested sites where a different color pattern morph of both R. variabilis and R. imitator can be found, the striped morph.
Journeying south of Tarapoto, one comes to the Huallaga River. At this time, the only way to cross was via ferry, a ferry that Rainer had helped build when he worked in the civil service! Here we are crossing the river with the unimog.
Once on the other side, one can drive up to a beautiful lake and the town of Sauce, a small resort town on the lake.
This area is home to Ranitomeya summersi (kindly named after me by my former students, Jason Brown and Evan Twomey), a close relative of R. fantastica. This species co-occurs with another morph of R. imitator, which closely resembles the color pattern of R. summersi. If one goes east along the Huallaga, the road goes to Chazuta, passing along some scary cliffs but providing beautiful views of the river. At certain times of the year, one will see congregations of people gathered to try to catch fish like the dorado catfish, which migrate upstream in the dry season. People will camp out for days, and build makeshift wooden platforms out into the river, to try to get access to the seething masses of fish making their way up the river. These kinds of migrations are amazing, and happen in various places in Amazonia, involving not just fish but other animals like caiman (secret caiman lake).
The end of the road is reached at Chazuta, but one can hire a boat here if one wants to go further down the Huallaga.
This is a fascinating trip – the scenery is beautiful and the frog color patterns seem to change before your very eyes. My first time down the river we hired a guide who had worked there for many years. In fact, he had worked there during the years in which narcotraficantes (drug lords) completely controlled the valley. He would bring bales of coca leaves down from the upper reaches of the valley, and exchange it for bales of money which he would take back up to pay the growers and narcotraficantes. That was during the days in which guerilla groups such as Tupac Amaru and the Shining Path controlled large swaths of Peru. When Alberto Fujimori became president he waged a successful war against the narcotraficantes and the guerillas, drastically curtailing their territory and influence. Fujimori himself was later convicted of corruption and sent to jail, but his campaign against the narcotraficantes was very effective.
Populations around Santa Rosa show the clear banded pattern like the Sauce population. But as we head down the river the populations start to change, the frogs begin to look different.
Ranitomeya imitator from the Huallaga Canyon. Photos: Evan Twomey

Further down the Huallaga, the R. imitator populations start to take on a distinctly striped appearance, very similar to the local striped R. variabilis (and to those near Pongo).
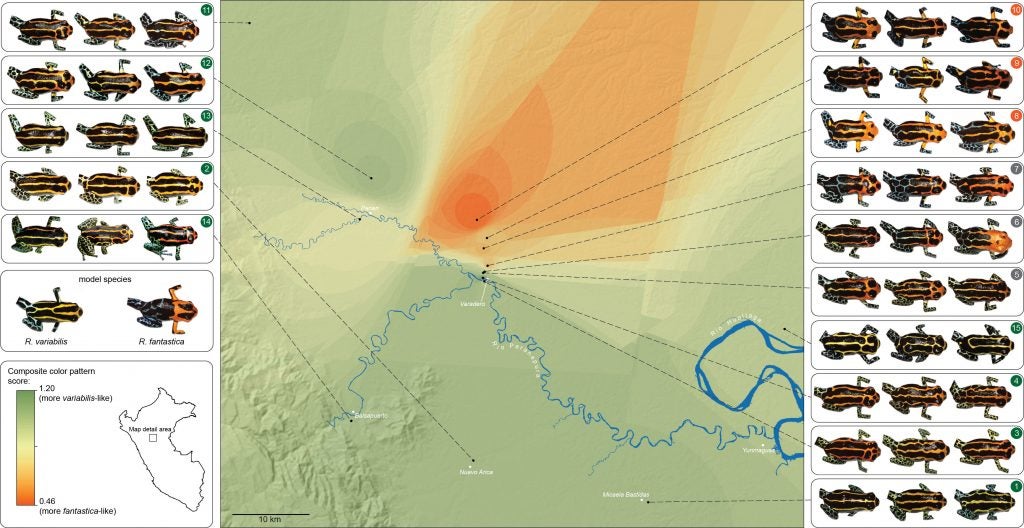
Another population of R. imitator, discovered near the village of Varadero by my former students, Jason Brown and Evan Twomey, closely resembles the local population of R. fantastica.
Evan Twomey (facing the camera) and Jason Brown heading upriver.
Peki-Peki boat
When we first started investigating these frogs, my graduate student, Beckie Symula (now a professor at the University of Mississippi (R. Symula webpage), and I, along with Rainer, decided to look at their evolutionary relationships, using molecular phylogenetic analyses (Symula et al. 1999). These analyses showed that the different color pattern morphs of R. imitator are all closely related, apparently having colonized norther Peru from the south, and evolving to resemble other species already there.
So, in summary, different populations of a single species, R. imitator, the mimic poison frog, resemble other species in different geographic regions. Different color morphs of R. imitator come into contact in some regions, resulting in “transition zones” with intermediate color patterns.
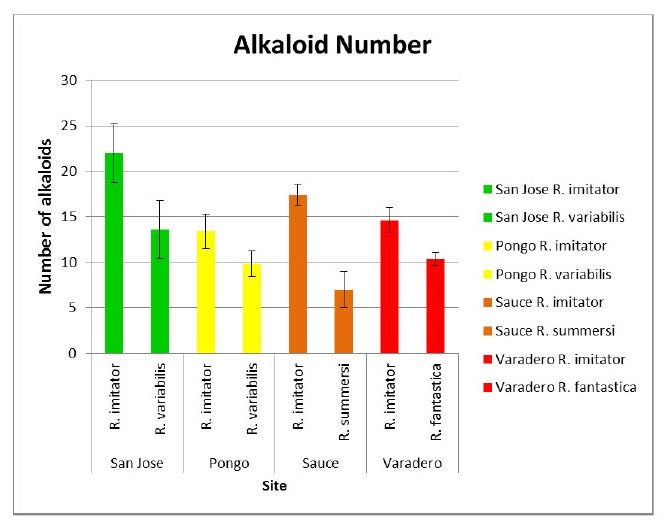
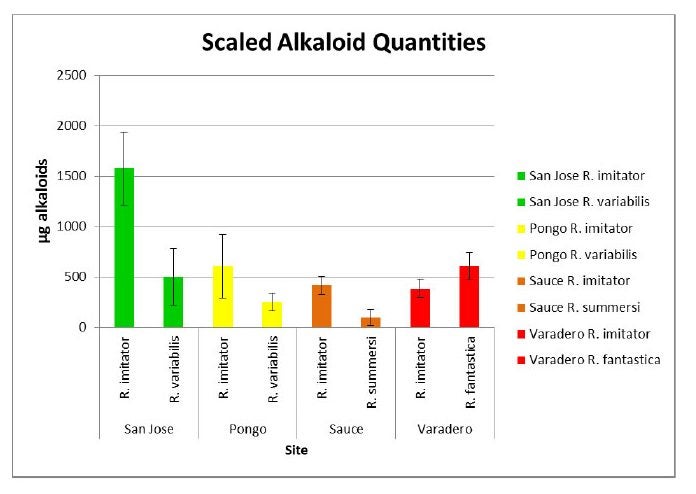
These frogs appear to be Mullerian mimics. In this form of mimicry, defended (e.g. toxic) species evolve to resemble each other. According to theory, the (defended) potential prey receive an advantage from this resemblance because predators only have to learn to avoid one color pattern rather than two. Since predators learn which animals to avoid by trial and error, and mistaken attacks impose a cost on even defended animals, defended species gain an advantage by training predators more efficiently if they share the same color pattern as other defended species (a form of mutualism). So, how do we know these frogs are mimics? My former grad student, Adam Stuckert (now an NSF postdoc at the University of New Hampshire), spent a considerable amount of time trying to address this question. First, he wanted to find out if all the species involved in this system were, in fact, toxic. To do this, he sampled R. imitator populations and the corresponding populations of the other species that shared the same morph (R. variabilis, R. fantastica and R. summersi) for skin toxins (Stuckert et al. 2014a).
As can be seen from the figures below, all the populations of each species contain a substantial diversity and quantity of toxins, as assumed by the theory of Mullerian mimcry.
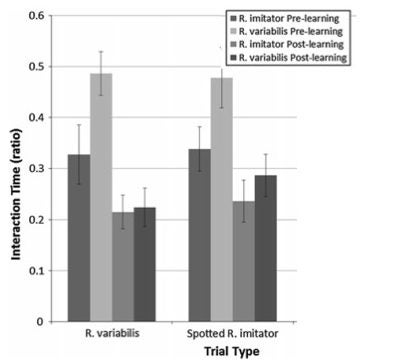
Next Adam wanted to see whether predators could that learned to avoid one species (through trial exposure) would also learn to avoid the other. He carried out experiments with chickens as model predators, exposing them to one species then checking their response to the other, and vice-versa (all in comparison to their response to non-toxic, undefended species of frog).
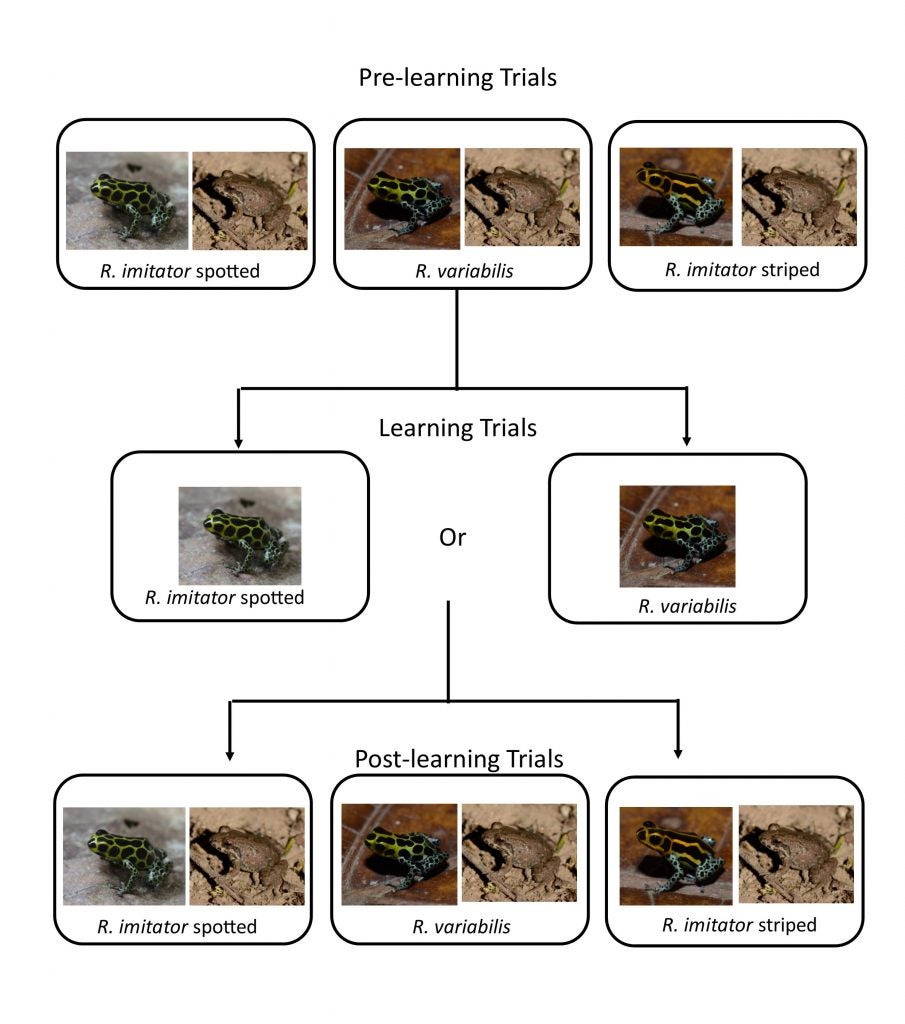
These trials demonstrated that predators (chickens) do learn to avoid one species when trained on the other, and vice versa.
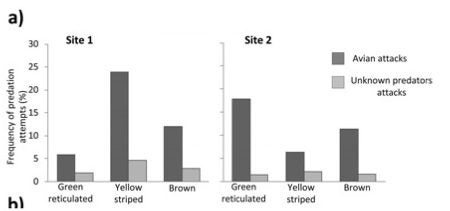
Another student and collaborator of mine, Mathieu Chouteau (now a research scientist at the CNRS in France)(M. Chouteau website) carried out key experiments on mimicry in R. imitator using clay models. He set out models of both the “correct” (i.e. local) color pattern morph and an “incorrect” color pattern (i.e. the color pattern from a different region), and vice-versa, in a reciprocal experiment. This work was published in the American Naturalist in 2011 (Chouteau & Angers 2011)
These experiments showed that predator (bird) attacks are more frequent on the “incorrect” (non-local) plasticene models, consistent with the idea that predators have learned to avoid the local color pattern morph.
Figure from Chouteau & Angers 2011
Mathieu and several others, including Justin Yeager (photo at left) (J. Yeager website) and Evan Twomey (below), carried out further analyses of genetic structure in the different populations of R. imitator, as described in these articles (Chouteau et al. 2011b)(Yeager et al. 2012)(Twomey et al. 2013).
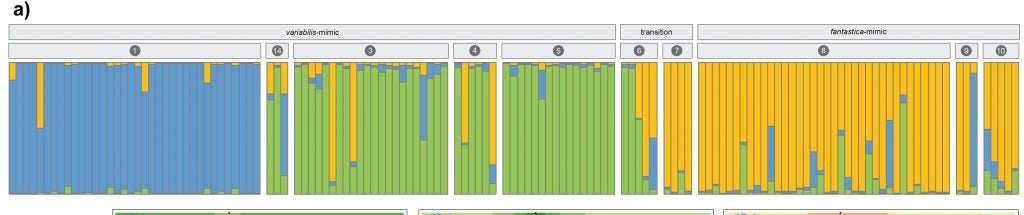
Given that selection is apparently driving different populations of R. imitator apart, is it possible that some of them may be diverging to the point of incipient speciation? My former student, Evan Twomey (Formerly at the University of Brussels (E. Twomey Webpage), now at Goethe University in Germany) undertook extensive research to address this question. In a series of papers, he investigated patterns of morphological, acoustic and genetic change across different color pattern transition zones (Twomey et al. 2014) (Twomey et al. 2016)
Evan collected samples from across the three main transition zones between morphs, including spectral reflectance (color) and tissues for genetic analyses.
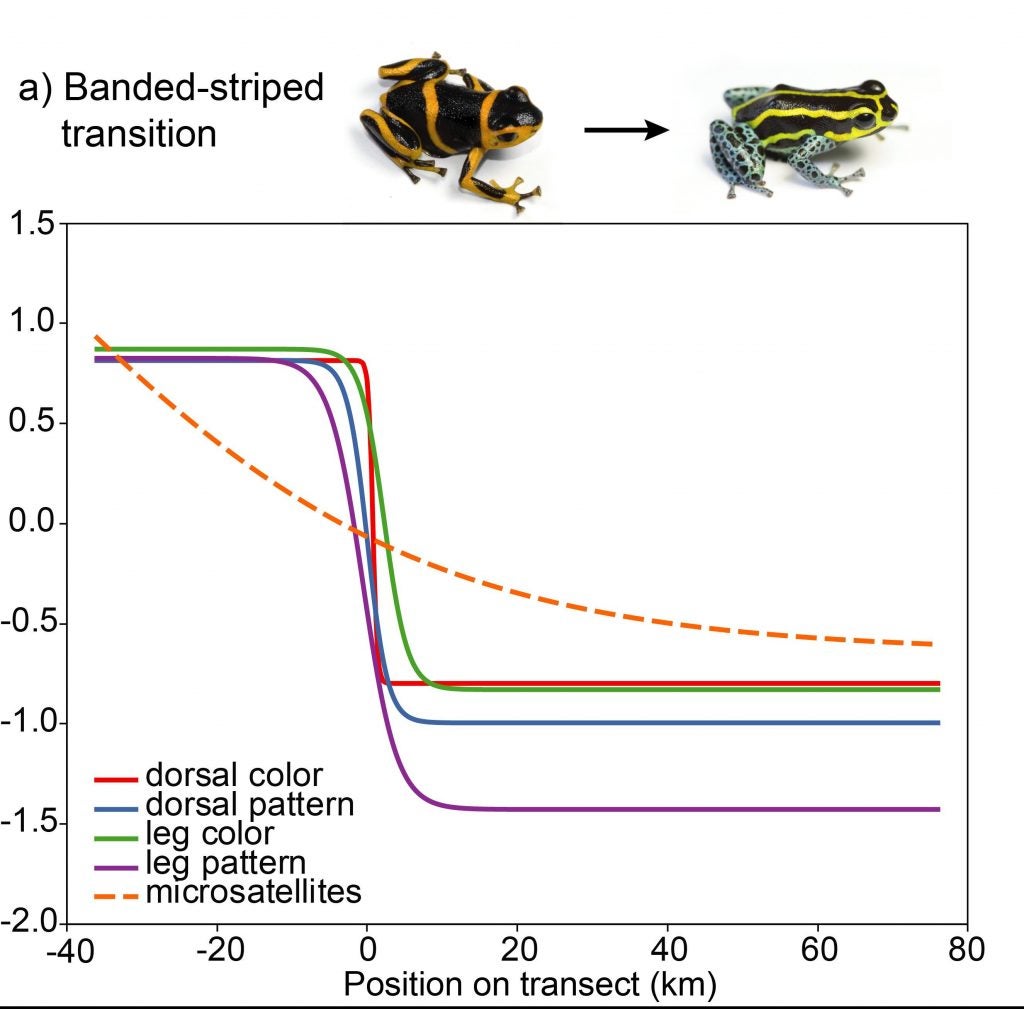
Evan found a clinal pattern of change across the transition zones in a number of different aspects of color and pattern, but not in genetic change.
The banded-striped morph transition showed no evidence for genetic structure, indicating high gene flow across this color pattern transition.
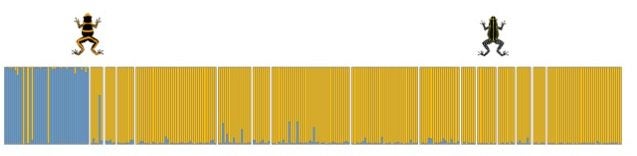
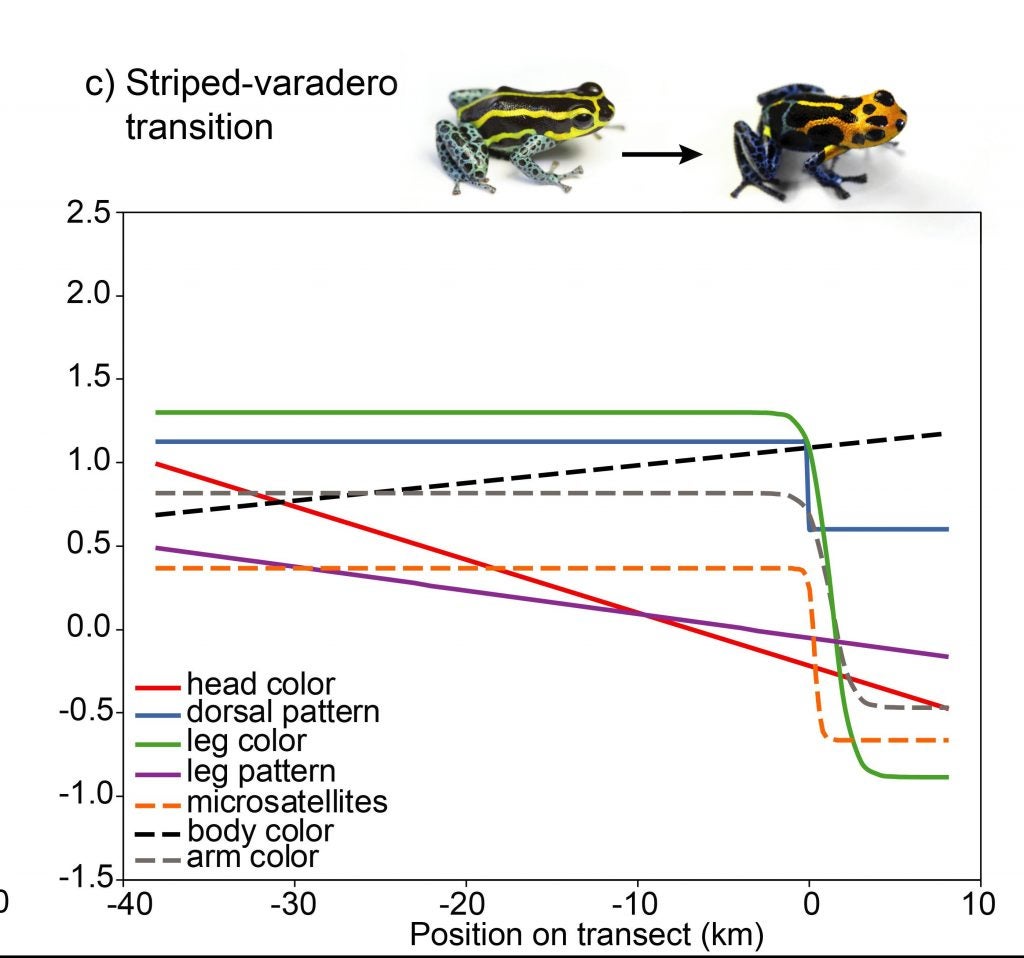
In contrast to samples from the banded-striped and spotted-striped transitions zones, the Varadero-striped transition zone showed more abrupt color pattern transitions over a short distance.
There is also an abrupt genetic change across this very narrow transition zone.
So, so in summary, there is a correspondence between morphological and genetic divergence, both of which fit a steep clinal model of change.
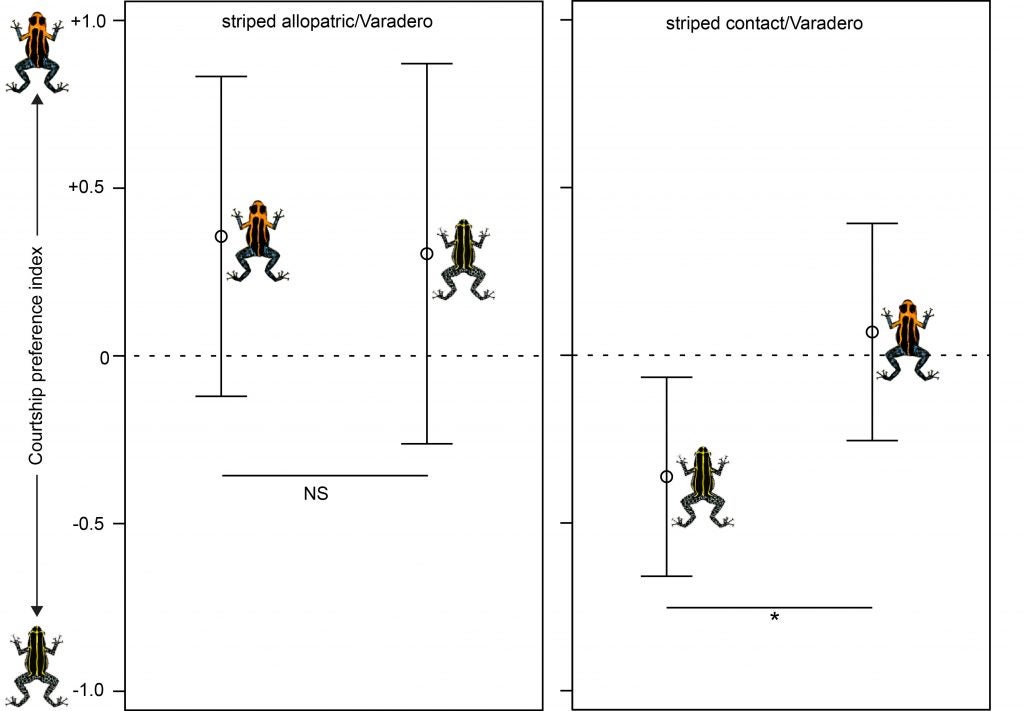
Evan also looked at reproductive isolation by carrying out experiments on mate preferences. These experiments demonstrated that striped R. imitator from the zone of sympatry demonstrated a significant preference for their own morph, whereas striped R. imitator from an allopatric (distant) population did not, consistent with the possibility that selection is driving reproductive isolation between these two color pattern morphs (Varadero and striped).
In summary, the Varadero population of R. imitator may be in an incipient stage of divergence on the way to speciation (Twomey et al. 2014) (Twomey et al. 2016)!
Jason and Evan think that mimicry may be quite widespread in this genus. Below is a montage that Jason created to illustrate the many different matching morphs that he has identified in this group.
What kind of genes underlie this rapid radiation in color pattern in R. imitator? This is a difficult problem to address because of the very large size of the genome in many amphibians. Unfortunately, poison frogs are no exception so we are forced to take indirect approaches to try to connect the genotype to the phenotype in these frogs. To try to identify these genes, we have been collaborating with the renown evolutionary geneticist, Rasmus Nielsen of UC Berkeley and his students (especially Tyler Linderoth and Jacob Vestergaard (Nielsen lab webpage)), and with Dr. Matthew MacManes of the University of New Hampshire (MacManes lab webpage).
Theoretical approaches developed by Dr. Nielsen and Dr. Vestergaard (in collaboration with Evan) gave us important insights in to the genetic basis of the changes in color pattern across the transition zones of R. imitator (for example, that specific aspects of color pattern in specific body regions are likely to be controlled by relatively small numbers of genes (Vestergaard et al. 2014).
More recently, we have begun to use genomic approaches, such as transcriptome sequencing and differential expression analyses, exome capture sequencing and admixture mapping, and genome sequencing, to identify the specific genes controlling color pattern variation (Linderoth et al. in prep; Stuckert et al. in prep).
Jason Lee Brown (now a professor at Southern Illinois University (Brown Lab Webpage)) began working in Peru in 2002 and spent many years studying the reproductive strategies of R. imitator and R. variabilis at fieldsites near Tarapoto. These two species provide a fascinating contrast because one has male-only parental care, whereas the other has biparental care.
Frogs have an incredible diversity of reproductive strategies, and many species (especially in the tropics) have evolved elaborate parental care strategies that enhance the survival prospects of their offspring. In the big picture of frog behavioral evolution, parental care is widely dispersed on the evolutionary tree, and largely restricted to tropical species. Life in the rainforest seems to have a liberating effect on frogs in terms of their reproductive modes. The availability of consistently moist habitat niches allowed tropical frogs to expand in to many different kinds of reproductive niches, including breeding in foam nests, burrows, leaf litter, tiny pools like bromeliad tanks, and even direct development. While predation was likely a key factor driving frogs to breed away from large bodies of water like pools and streams (link), the humidity and rainfall (and warm temperatures) characteristic of tropical rainforests was what allowed frogs the freedom to find and exploit breeding niches away from ponds and streams. Yet the use of such sites often favors small clutch sizes and extended parental care of eggs and even tadpoles. When tadpoles are placed in extremely small water bodies (such as pools that form in plant leaf axils), the availability of nutrients may be so low that parents do best by feeding the offspring in these tiny pools.
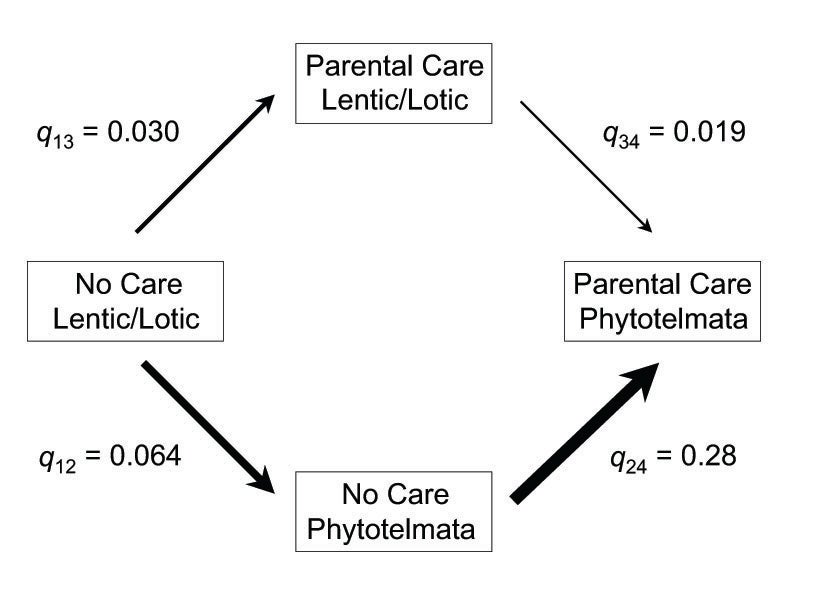
Based on a reconstruction of the evolution of parental care and breeding pool use across an evolutionary tree of frogs (about 500 species), the evolution of parental care in frogs is correlated with the use of smaller pools (Brown et al. 2010).
Directional analyses of evolutionary change indicate (not surprisingly) that pond/stream breeding is the ancestral condition for frogs, and that changes in the size of pools used were associated with the evolution of parental care (Brown et al. 2010).
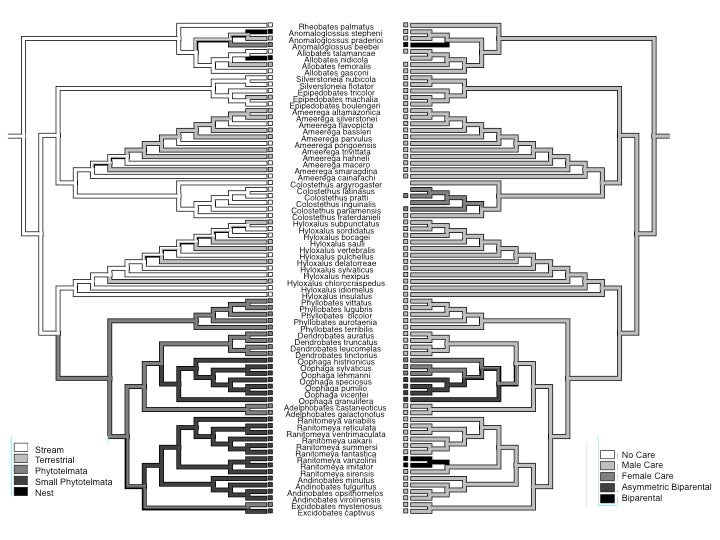
The Neotropical poison frogs are famous for their diversity of reproductive strategies. A number of species in genera like Colostethus, Hyloxalus, Epipedobates and Amereega lay their eggs on leaves on the ground, but then carry their tadpoles to streams or small terrestrial poos (such as those that form in fallen palm fronds).
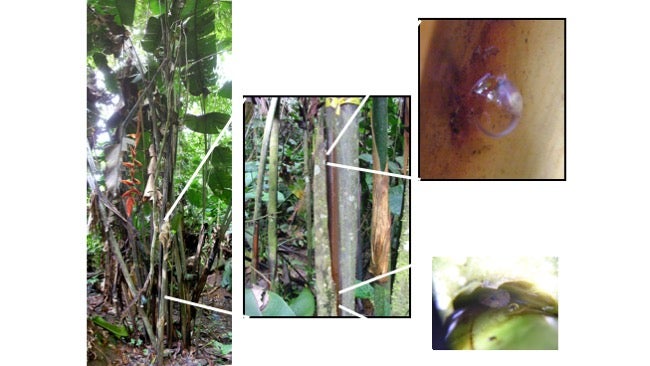
(A) A male Hyloxalus nexipus attends an egg clutch of ∼16 eggs laid on a leaf on the forest floor, and (B) transports all the tadpoles from one clutch on his back. (C) A male H. nexipus with tadpoles next to a flowing stream where the tadpoles will be deposited. (D–F) Many members of the genus Ameerega are classified as terrestrial breeders. (D) A male A. hahneli attends a clutch of seven eggs, and (E) transports them once they hatch. (F) A male A. bassleri deposits tadpoles in a terrestrial pool; arrows indicate tadpoles already in the water. Photographs courtesy of Jason Brown (B, D), Adam Stuckert (A, E), and Evan Twomey (C, F).
Species in genera like Dendrobates, Ranitomeya and Oophaga use tiny pools that form in the leaf or stem axils of plants (phytotelmata).
(A) A male Ranitomeya variabilis transports three tadpoles that will be deposited in phytotelmata such as bromeliad axils. Similar to terrestrial- and stream-breeders, no further care is provided after tadpole deposition. (B) A male R. imitator transports each tadpole from a clutch, individually, to small, nutrient-poor phytotelmata. (C) After tadpole deposition the male calls to the female, leads her to the pools occupied by their offspring, and continues to call, stimulating her to provision tadpoles with unfertilized trophic eggs. See color plate at the back of the book. Photographs courtesy of Jason Brown (A), James Tumulty (B), and Adam Stuckert (C).
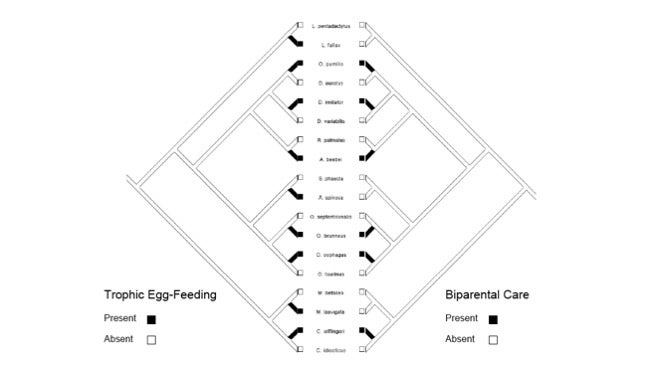
If we look specifically at the Neotropical poison frogs, the evolution the use of smaller pools is correlated with the evolution of more intensive parental care strategies (e.g. biparental care and egg (Summers & Tumulty 2014)
Also, the evolution of biparental care is significantly associated with the evolution of trophic egg-feeding (Brown et al. 2010).
Ranitomeya imitator breeds in extremely small pools, such as those that form in the leaf axils of Heliconia plants. These pools are tiny and nutrient poor. Ranitomeya imitator parents intensively care for their offspring after they are placed in these tiny pools, frequently revisiting them and providing them with trophic eggs (Brown et al. 2008).
In contrast, Ranitomeya variabilis, while still breeding in phytotelmata, use larger pools and do not feed their offspring.
Observational and experimental evidence indicate that the use of these very tiny, nutrient-poor pools by R. imitator has led to a variety of key life history changes (Brown et al. 2008) , including the evolution co-defended territories (Brown et al. 2009) changes in pool preferences and tadpole aggressiveness and competitiveness (Brown et al. 2008b)(Brown et al. 2009) and to intensive biparental care, pair-bonding and monogamy (Brown et al. 2010).
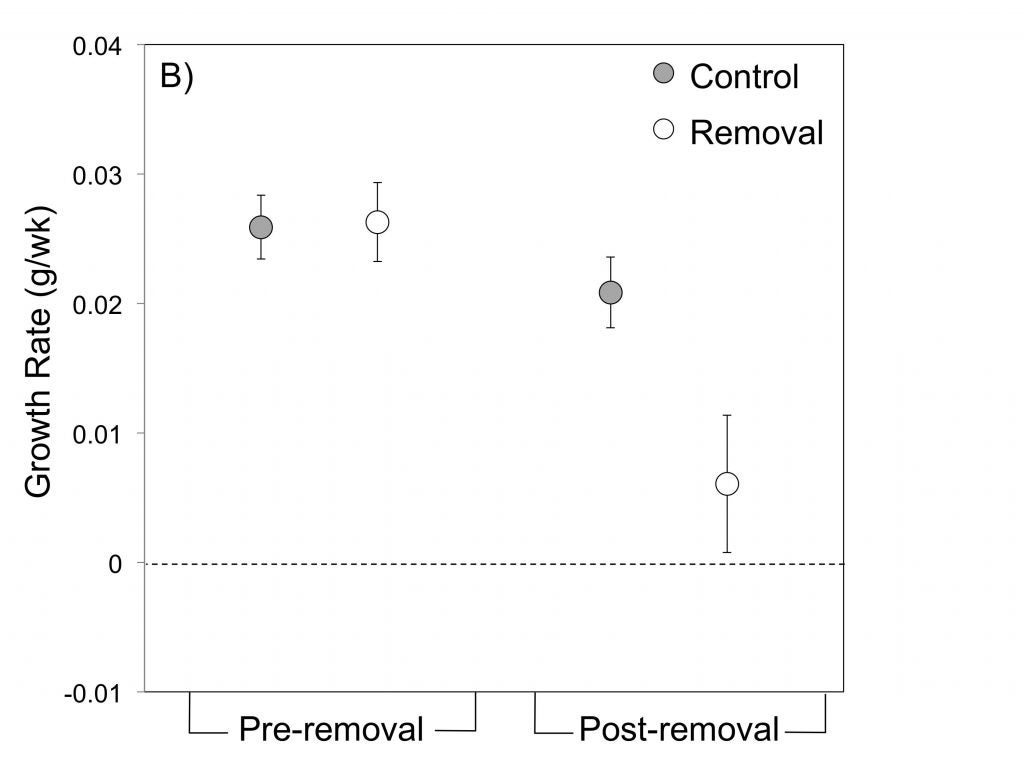
For example, field experiments demonstrated that tadpoles (or either species) cannot grow and survive in the small pools used by R. imitator without supplemental feeding (Brown et al. 2010). Hence, female parental care (egg-feeding) is crucial to offspring survival in R. imitator.
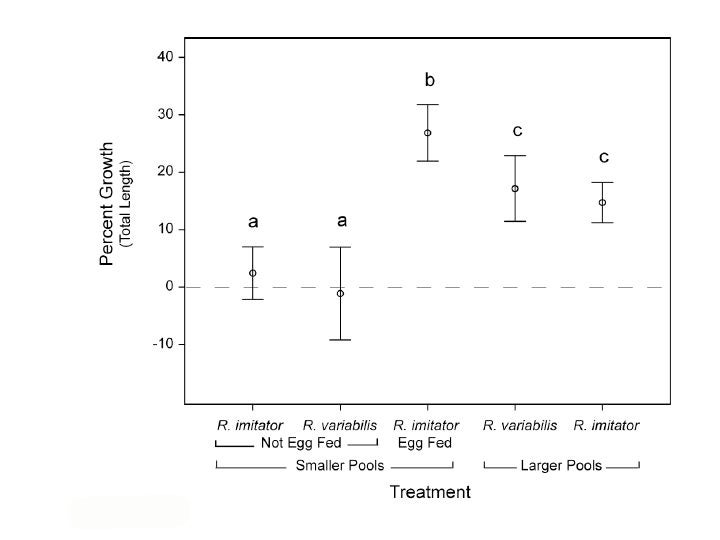
In Summary, R. imitator appears to have evolved pair-bonding, intensive biparental care and monogamy in concert with a shift to the use of extremely small, nutrient-poor pools (Brown et al. 2010).
Mate removal experiments by James Tumulty (Tumulty et al. 2014) demonstrated that male care, as well, is critical to tadpole growth and survival in R. imitator, confirming the key importance of biparental care to R. imitator reproductive success, and supporting the hypothesis that the need for biparental care favors the evolution of pair-bonding and monogamy.